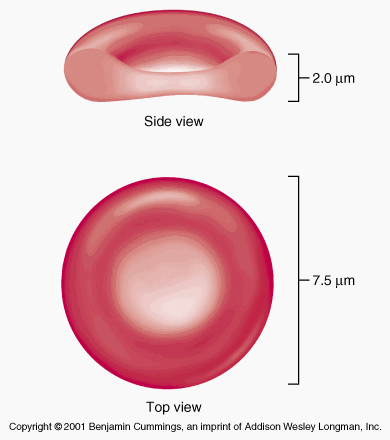
Red blood cells, so nice and simple. It’s literally a red, blood cell. Amazing. 10/10 would study. Clearly we can all appreciate the normal shape of a red blood cell:
- The key word for it is biconcave disc, meaning that it is a disc shaped (or circular) cell that is compressed at the centre in both directions. This allows an increased surface area: volume ratio, providing easier avenues for diffusion of oxygen.
- They have a typical diameter of 7.2um, however the normal range is between 6.2um and 8.2um.
- They have a typical width of 2.0um, although it can range between 2.0um and 2.5um.
- They contain no nucleus, to maximize volume of Hb so as to maximize carriage of oxygen.
- They are also very flexible, useful for squeezing into thin capillaries to deliver oxygen to tissues.
Great, that’s all gu- wait.. morphological abnormalities? …Oh man, so much can go wrong with red blood cells. Oh boy, here we go.
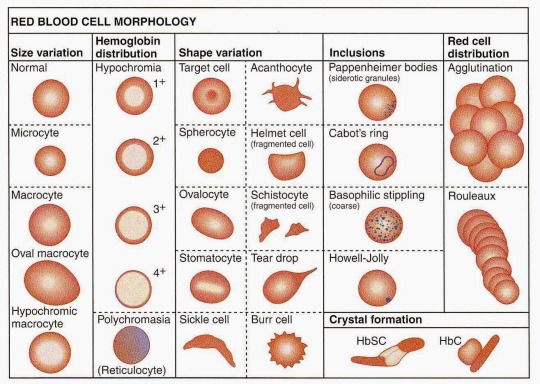
Yep, all these things can go wrong with red blood cells. Poor things. There are actually more things than these, but let’s go through these in detail.
Variations in Size
We have mentioned under “General Features of Anemia” that the most important red cell index for discussing the variations in size of blood cells is the Mean Corpuscular Volume (MCV). Just to refresh, the MCV is the average volume of a red blood cell. It is reflective of the size of a cell, and thus, a large MCV indicates a large red blood cell, and a small MCV indicates a small red blood cell. Its typical reference value is 80fL – 95fL, though some sources use 100fL as the upper limit.
Any variation in size of the RBCs is known as anisocytosis, and the degree of anisocytosis in a sample of blood is known as the red cell distribution width (RDW).
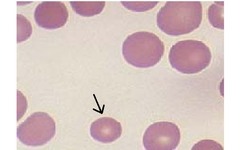
Microcyte
A microcyte, is a red blood cell by definition, a small (micro-) mature cell (-cyte). Thank you for making it so simple, greek derivations. In terms of MCV, a microcyte has an MCV below 80fL. Since the reference range of MCV is 80-95, this should be easy to remember.
In terms of actual diameter, a microcyte is defined as any RBC with a diameter less than 5.0 microns. Compare this to the average of 7.2 microns.
Diseases Associated with Microcytes:
Microcytes indicate some problem with the manufacturing system of red blood cells, for example, by some deficiency. The diseases include:
- Iron Deficiency Anemia
- Deficiency of iron leads to a scenario where the red blood cell cannot be filled with Hb and thus is an overall smaller cell, since there is less Hb.
- Sideroblastic Anemia
- Condition where there the bone marrow releases immature red blood cells, sideroblasts, as opposed to mature red blood cells.
- These sideroblasts have rings of iron around their nucleus, and thus the iron is used up on the sideroblasts.
- Thus, for congenital forms of sideroblastic anemia, there is often a defiency of iron leading to red blood cells that do mature, becoming microcytes.
- Thalassemia Minor
- Beta thalassemias are a group of disorders in which the beta chain of Hb is usually missing or deformed.
- They occur as a result of blockage to the HBB gene.
- HBB blockage over time leads to decreased Beta-chain synthesis. The body’s inability to construct new Beta-chains leads to the underproduction of HBA. Reductions in HBA available overall to fill the red blood cells in turn leads to microcytic anemia.
- Lead Poisoning
- One of the main causes for the pathology of lead is that it interferes with the activity of an essential enzyme called delta-aminolevulinic acid dehydratase, or ALAD, which is important in the biosynthesis of heme, the cofactor found in hemoglobin. Lead also inhibits the enzyme ferrochelatase, another enzyme involved in the formation of heme.
- The important thing to understand is that microcytosis in lead poisoning occurs due to lack of heme, which leads to a lack of Hb and thus a deficiency, causing smaller RBCs.
- Some Hemoglobinopathies
- Occasionally, chronic disease
Here is a microcyte below:
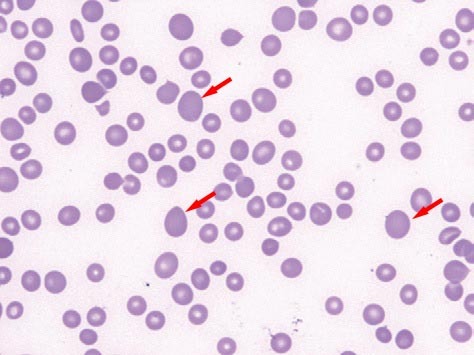
Macrocyte
A macrocyte on the other hand, is a large (macro-) mature cell (-cyte). Wow. In terms of MCV, a macrocyte has an MCV above 95, or above the reference range. It is thus an enlargement of red blood cells with a near constant concentration of hemoglobin.
Diseases/Conditions associated with Macrocytes:
- Liver Disease
- Patients with hepatic disease and obstructive jaundice have macrocytosis that is secondary to increased deposition of cholesterol or phospholipids on the membranes of circulating red blood cells (RBCs).
- Megaloblastic Anemia
- The most common cause of macrocytic anemia is megaloblastic anemia, which is the result of impaired DNA synthesis. Typically, although DNA synthesis is impaired, RNA synthesis is not, and RNA continues to be produced, increasing the nuclear matter within RBCs that is not being converted to DNA. Thus, the cell gradually enlarges due to increased nuclear matter, causing macrocytosis.
- Vitamin B12 deficiency and Folate deficiency
- Vitamin B12 and folate is very important in the synthesis of Thymidine and Purines, and thus DNA synthesis is impaired in these deficiencies, causing macrocytosis by the aforementioned mechanisms.
- Aplastic Anemia
- Mild macrocytosis is seen in recovery from aplastic anemia, which occurs when there is a bone marrow pathology and a deficiency of all 3 types of blood cells (WBC, RBC, platelets) occurs.
- Neonates
Observe macrocytes below:
Notes about Red Cell Distribution Width (RDW)
RDW is the most important index relating to anisocytosis. The higher the variance in sizes of cells, the higher the RDW. Thus, a high RDW means that cells are of very different sizes, whereas a low RDW indicates that cells are more or less of the same size.
With this in mind, the normal reference range in human red blood cells is 11.5-14.5%, meaning that usually, only 11.5% – 14.5% of cells are of different sizes out of a sample of blood.
Implications of RDW:
Normal RDW in presence of Anemia: A normal RDW implies that most cells are of the same size. In cases of a normal RDW with anemia, the diagnosis is almost exclusively a thalassemia. This is since beta chains are gradually removed, and the cells become smaller, all the RBCs drop to smaller sizes, by the mechanism explained above. All the cells are affected since it is a genetic disorder. Since all the cells are now microcytic, they are expected to be of similar sizes, and thus the RDW remains normal.
Abnormally High RDW: An abnormally high RDW on the other hand means that cells are of a variety of different sizes. High RDWs are generally expected to occur in situations of deficiencies, since only some cells will be deficient and thus affected at a time. Notice that this is different from genetic conditions, where ALL cells are affected. High RDWs are usually indicative of:
Iron Deficiency Anemia: High RDW and Low MCV
Vitamin B12 and Folate Deficiency: High RDW and High MCV
Recent Hemorrhage: High RDW and normal MCV
Variations in Colour
Remember that the red blood cell consists of hemoglobin that can be in two states: Either oxygenated, in which case it is oxyhemoglobin, or deoxygenated, in which case it is deoxyhemoglobin. Well, the color of a red blood cell is… red. Yes. I don’t know where I was going with that.
To be more precise, oxyhemoglobin and deoxyhemoglobin are different shades of red. Oxyhemoglobin is a brilliant scarlet, while deoxyhemoglobin is a darker burgundy-red.
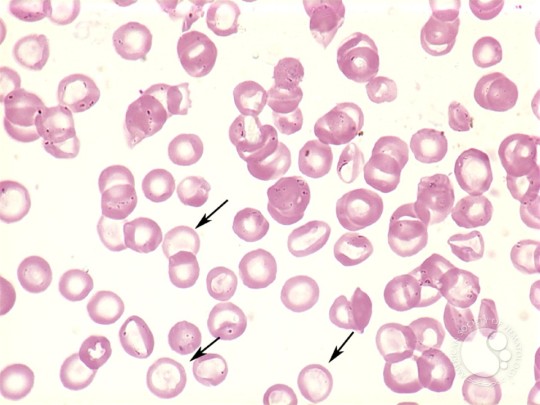
Hypochromia
Hypochromia is the most common disorder of color that occurs in red blood cells. Hypo-, of course, means “less” and -chromic means “color” so when we describe a cell as hypochromic, we say it has less color. Simple, right?
Hypochromic RBCs are much paler, and the reason for this is because they lack hemoglobin within them. Because of this, they lose their red colouration and appear much paler. Using this concept, recall that the unit to measure the amount of hemoglobin per cell is mean corpuscular hemoglobin, or MCH. The MCH is simply the average amount of hemoglobin in one red blood cell, from a particular sample. Its reference range is 27pg – 31pg, meaning that there is typically between 27pg and 31pg of hemoglobin in a single red blood cell.
The other unit for hemoglobin content of a red blood cell is mean corpuscular hemoglobin concentration (MCHC). The MCHC is reflective of the concentration of packed red blood cells (so blood excluding plasma) that is hemoglobin. It basically translates to the amount of hemoglobin present in the cellular component of blood, and thus excluding plasma. Its reference range is 32g/dL – 36g/dL.
There are two conditions that a RBC must satisfy in order to be classified as a hypochromic cell. These are:
- The central zone of pallor of the RBC must be greater than 1/3 of the diameter of the cell.
- The MCH must be below 27pg/cell and/or the MCHC must be below 32g/dL.
Diseases associated with Hypochromia:
- Iron Deficiency Anemia
- Recall that iron is essential in the production of heme, the centerpiece of hemoglobin.
- Thus a deficiency in iron leads to a deficiency in heme and thus hemoglobin, resulting in lowered MCH values.
- Also recall that iron deficiency anemia causes microcytosis, and thus iron deficiency anemia causes hypochromic, microcytic anemia.
- Thalassemias
- As discussed previously, thalassemia arises by a genetic defect in the HBB gene that codes for beta chains of hemoglobin, causing the absence of, or underproduction of, beta chains. As alpha chains production gradually slows down in the absence of beta chains, hemoglobin becomes less and less functional. Thus, less oxygen can be held by hemoglobin, and hemoglobin itself becomes destroyed due to destruction of the then non-functional red blood cells by thalassemia. Consequently, MCH decreases and hypochromic anemia results.
- Notice that thalassemia also causes microcytosis as HBA production to replace HBB decreases over time and the red blood cell shrinks.
- Sideroblastic Anemias
- Recall that sideroblast production (very immature red blood cells), with rings of iron around their nucleus is the hallmark of sideroblastic anemias. These cells are known as ringed sideroblasts.
- Because iron is used up in these non-functional rings, proper red blood cells are not formed, and thus proper hemoglobin is not formed adequately. This results in hypochromic cells, that are again, also microcytic.
- Lead Poisoning
- Lead poisoning impairs the functioning of the enzyme, ALAD, and the enzyme ferrochelatase, both important in the production of heme.
- This impairment in the production of heme leads to decreased hemoglobin in red blood cells, thereby reducing MCH and MHCH and causing hypochromia and microcytosis.
- Some cases of chronic inflammation
Polychromasia
Polychromasia is a medical condition in which there is an abnormally high amount of immature red blood cells being released into the bloodstream. The most significant of these is the reticulocyte, the immediate precursor to the red blood cell. The only difference between the reticulocyte and the red blood cell is the presence of a meshwork of RNA within the reticulocyte, when viewed with special stains such as the new methylene blue stain, that must be removed before the reticulocyte can be called a red blood cell.
Polychromasia literally translates to “many colors,” and the reason for this is because the many immature red blood cells being released into the bloodstream are all different shades of a bluish grey.
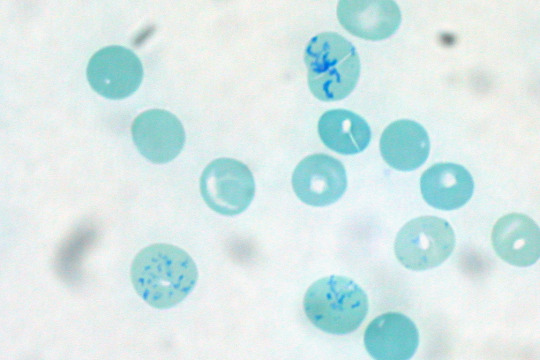
Man, that looks like a great color for hipst-, ahem, anyways.
Diseases Associated with Polychromasia:
Polychromasia is usually a sign of bone marrow stress as well as immature red blood cells. Polychromasia occurs in conditions which call for premature release of red blood cells into the bloodstream, such as in conditions where RBC levels in the bloodstream are severely low. These include:
- Acute and Chronic Hemorrhage
- During hemorrhage, as blood loss from the body occurs, there is a constantly lowered level of RBCs in the bloodstream. Thus the bone marrow opts to increase the RBC levels by increasing production of red blood cells, and increasing release of red blood cells, even if they are immature or not fully mature. In this way, immature cells such as reticulocytes may appear in the bloodstream, causing polychromasia.
- Hemolysis
- In hemolysis, as red blood cells are destroyed, the body attempts to replace them by increasing production and release of red blood cells, and this results in the release of immature red blood cells such as reticulocytes into the bloodstream.
- Effective treatment for anemia
- Neonates
Note that polychromasia is mainly associated with normocytic styles of anemia, as there is no change in MCH or MCV in any of these scenarios.
Variations in Shape of Cells
The term for variations in the shape of cells is poikilocytosis. Thus, any abnormally shaped cell is called a poikilocyte.
Poikilocytes can occur either due to membrane abnormalities or trauma.
The poikilocytes caused by membrane abnormalities are:
- Acanthocytes (Spur Cells)
- Codocytes (Target Cells)
- Echinocytes and Burr Cells
- Spherocytes
- Stomatocytes (Mouth Cells)
- Drepanocytes (Sickle cells)
- Degmacytes (”Bite Cells”)
The poikilocytes caused by trauma are:
- Dacrocytes (Teardrop cells)
- Keratocytes
- Microspherocytes and Pyropoikilocytes
- Schistocytes
- Semilunar Bodies
Let us explore each in detail.
Poikilocytes of Membrane Abnormalities
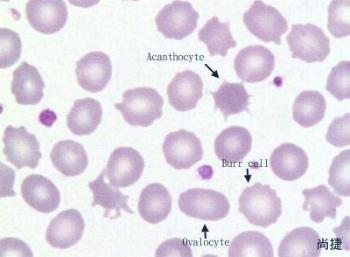
Acanthocytes (Spur Cells)
The word acantho- means thorns. This should give you a good idea as to what acanthocytes look like. The word literally means thorn cells after all. Thus, acanthocytes can be described as having a spiked cell membrane, due to irregular thorny projections that vary in width, length and number. Notably, they have no central area of pallor.
Acanthocytes typically arise via one of two mechanisms: Alterations in membrane lipids are seen in abetalipoproteinemia and liver dysfunction.
- In liver dysfunction, apolipoprotein A-II deficient lipoprotein accumulates in plasma causing increased cholesterol in the RBC membrane. This causes abnormalities of membrane of RBC causing remodeling in spleen and formation of acanthocytes.
- In abetalipoproteinemia, there is deficiency of lipids and Vitamin E causing abnormal morphology of RBC membranes.
Diseases Associated with Acanthocytes:
- Abetalipoproteinemia
- This is a rare autosomal recessive condition that affects the absorption of fat, and fat soluble vitamins from food.
- Abetalipoproteinemia affects the absorption of dietary fats, cholesterol, and certain vitamins. People affected by this disorder are not able to make certain lipoproteins (hence the name, abetalipoproteinemia).
- This leads to a multiple vitamin deficiency, affecting the fat-soluble vitamin A, vitamin D, vitamin E, and vitamin K. However, many of the observed effects are due to vitamin E deficiency in particular.
- As explained above, the deficiency of Vitamin E and lipids results in an abnormal membrane layer of the RBC.
- Abnormal lipid concentrations within the blood cause acanthocytosis primarily by inducing concentration gradients with the lipids in the red cell membrane, causing some portions of the membrane to extend outwards as lipids move in or out of them. This gradient is known as membrane stress, and in conditions of abetalipoproteinemia or hypobetalipoproteinemia, the RBC membrane is more vulnerable to membrane stress.
- Vitamin E Deficiency
- Severe Liver Disease
- As explained above, cholesterol buildup in RBCs causes portions of the red cell membrane to extend out the RBC and form thorny extensions, thus producing acanthocytosis.
- Splenectomy
- A major function of the spleen is the clearance of opsonized, deformed, and damaged erythrocytes by splenic macrophages. If splenic macrophage function is abnormal or absent because of splenectomy, altered erythrocytes will not be removed from the circulation efficiently.
- Malabsorption
- Poor reabsorption of lipids and lipid-soluble vitamins has similar effects on RBCs as abetalipoproteinemia.
- Hypothyroidism
- Very rare cause of acanthocytosis.
- Neuroacanthocytosis
- This is a condition including 4 diseases, namely chorea acanthocytosis, McLeod Syndrome, Huntington Disease – like 2 (HDL2) and pantothenate kinase-associated neurodegeneration.
- In summary, all neuroacanthocytoses affect the basal ganglia and brain and are a group of movement and neurological disorders.
- All 4 conditions produce acanthocytosis (it’s in the name after all).
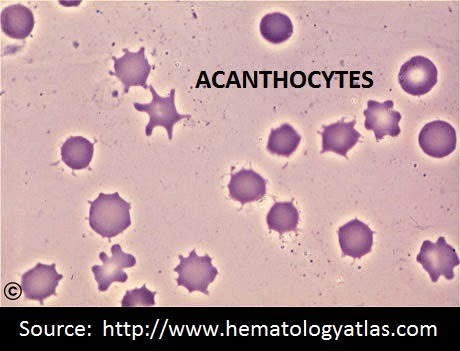
Notice the thorn like projections on each cell. They are irregular, and of varying distribution, length and width.
Codocytes (Target Cells)
Codocytes, also known as target cells, look like typical red blood cells, with a central area of pallor and appropriate size. However, they have a dark, red spot in the middle. For this purpose, they are also referred to as bullseye cells.
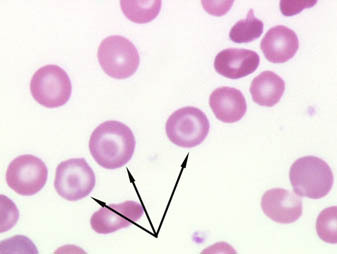
Yep, they look like bullseyes, don’t they? You can describe them of consisting of a central darkened area, surrounded by a white ring (or an area of achromia), in turn surrounded by a peripheral darkened stained area. For this reason, they are also called… Mexican Hat cells. …Yeah. Unforgettable now isn’t it?
They occur due to a disproportional increase in the surface area:volume ratio of a red blood cell. You can say these cells have an abnormally high surface area for their volume. It is due to either increased red cell surface area (increased beyond normal), or else a decreased intracellular hemoglobin content (which may cause an abnormal decrease in cell volume without affecting the amount of membrane area).
As a result of this increased surface area, these cells are stronger in water, and have decreased osmotic fragility. This is since they have a higher surface area for diffusion of water, they can take up more water for a given amount of osmotic stress.
It should be noted however, that within the blood vessels itself, the codocyte is in fact thin and bell-shaped, and occasionally the central darkened spot is connected to the peripheral darkened ring. This leads to the central zone of pallor being C-shaped. It is only when a blood film is made that the bell-shape is squashed and appears as a target. Thus, within the blood cells, since they appear different from target cells and codocytes, they are sometimes referred to separately as leptocytes.
Diseases Associated with Codocytes:
Diseases associated with codocytes must obviously cause at least one of two things: Either cause a direct increase in surface area by affecting lipid concentrations in the RBC membrane, or by decreasing hemoglobin concentration within the RBCs. Thus, any disease that can cause these two can cause codocytes to be formed. A list of some of these diseases includes:
- Thalassemia
- Associated with a decrease in functional hemoglobin.
- Iron Deficiency Anemia
- Associated with a decrease in total hemoglobin.
- LCAT Deficiency
- LCAT, or lechitin-cholesterol acyltransferase is an enzyme that coverts free cholesterol into cholesteryl ester. In the absence of LCAT, the cholesterol:phospholipid ratio increases, causing cholesterol buildup in the RBC and increasing the size of the membrane of the RBC. Eventually, the surface area rises to abnormally high levels.
- Obstructive Liver Disease
- Obstructive liver disease is associated with an LCAT deficiency.
- Hemoglobin C (and sometimes Hemoglobin S) Disease – Hemoglobinopathies
- In hemoglobiopathies such as Hemoglobin C and Hemoglobin S (sickle cell anemia) disease, one of the chains in hemoglobin is genetically malformed (contrast with Thalassemias where a chain is underproduced or missing). As a result of this, typically the hemoglobin is reduced and non-functional, and it causes red blood cells to have a shortened lifespan.
- Thus, codocytes are very typical of all hemoglobinopathies.
- Splenectomy
- Associated with removing opsonized of damaged cells, and thus target cells remain in the bloodstream and are not removed.
Echinocytes or Burr Cells
Echinocytes. Another type of red blood cell disorder. As usual, let’s allow our Greek friends to help us out. Echino- arises from the greek word echinos, which means hedgehog, or sea urchin. Let’s look at a hedgehog and see why echinocytes are named after them.

Wait no, wrong hedgehog.

Look at those spikes, quite regular and quite short. Echinocytes are actually quite similar.
Echninocytes are burr-like erythrocyte with short, blunt, evenly spaced projections. It is a red blood cell with an abnormal membrane, that, like acanthocytes, has thorny projections. The main difference between the acanthocyte and the echinocyte however, is the shape of the thorny projections. In acanthocytes, the projections are irregularly spaced, and vary in width, length and number. In echinocytes, the projections are all short and evenly spaced. Furthermore, under the Wright Stain, echinocytes even appear to have a central area of pallor. Acanthocytes show little to no central pallor.
Both acanthocytes and echinocytes occur due to membrane abnormalities of the RBC. Recall that acanthocytes arise from either increased cholesterol buildup within the membranes, or general lipid malabsorption that causes increased membrane tension, which is what leads to the irregular projections on its surface.
Echinocytes on the other hand can be produced in vitro by incubation at high pH or in the presence of high calcium concentrations, exposure to glass surfaces, reduced albumin concentrations, and after prolonged storage, and are usually reversible creations. They can be formed during application of EDTA, drying or staining.
They may also occur in hyperlipidemias caused by liver disease, as with acanthocytes. However, the cholesterol does not become incorporated into the lipid membrane as it does with acanthocytes. Instead, it is speculated that cell surface receptors on the red blood cells bind with HDL cholesterol which induces the shape change in echinocytes.
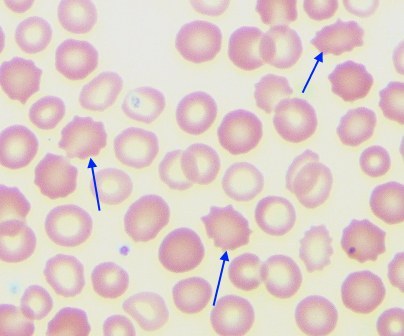
Echinocytes are seen above. Notice how their thorny projections are short and regular, and have a central area of pallor. Look at how they compare to acanthocytes below:
The acanthocyte thorns are much sharper, longer, random and show no central area of pallor, as opposed to the echinocyte (Burr Cell) that has short, round, very regular thorny projections and show a central area of pallor.
Diseases/Conditions Associated with Echinocytes
- Uremia
- Pyruvate Kinase Deficiency
- Microangiopathic Hemolytic Anemia
- Neonates (especially premature)
- As artifacts of EDTA, drying or staining.
Spherocytes
We’ve really been helped out here. With a name like “spherocytes” there’s no question what the shape of these cells will be. Spherical cells. Yeah. These cells are sphere-shaped rather than the typical biconcave disc shape expected of a normal red blood cell, and that is their most important feature.
Spherocytes are simple. They appear as spheres under a blood film with no central area of pallor.
You’ll notice that macrocytes also have no central area of pallor. To differentiate one from another, you’ll notice that the spherocytes are actually smaller than the normal red blood cells, and thus cannot be macrocytes. In contrast, microcytes show a central area of pallor, and thus you should be able to tell the difference between the three. The spherocytes are smaller due to partial loss of their membrane, without any damage to their intracellular content. The mechanism will be seen below.
Spherocytes are often always indicative of either hereditary spherocytosis or immune-mediated hemolytic anemia.
Hereditary spherocytosis is a genetic disorder, a molecular defect in one or more of the proteins of the red blood cell cytoskeleton, including, spectrin, ankyrin, Band 3, or Protein 4.2. Because the cell skeleton has a defect, the blood cell contracts to its most surface-tension efficient and least flexible configuration, a sphere.
Allternatively, during immune-mediated hemolytic anemia, there is partial phagocytosis of normal red blood cells by phagocytosis due to the presence of antigens on the surface of the red blood cell. The phagocyte destroys the membrane where the antigen was present, destroying some of the surface area of the cell. Thus, the cell must now shape itself into a sphere, decreasing its surface area:volume ratio, since the volume of the cell remains the same. This is known as immune-mediated hemolytic anemia, since the immune system in the form of phagocytes destroys (hemolyses) a portion of the red blood cells, producing spherocytes. Two simple mechanisms.
Though the spherocytes have a smaller surface area through which oxygen and carbon dioxide can be exchanged, they in themselves perform adequately to maintain healthy oxygen supplies. However, they have a high osmotic fragility–when placed into water, they are more likely to burst than normal red blood cells.
Diseases Associated with Spherocytes:
- Hereditary Spherocytosis
- Immune-mediated Hemolytic Anemia
- Haemolytic jaundice of the newborn due to ABO antibodies
- Transfused Cells
- Severe Burns
Stomatocytes (Mouth Cells)
Stomatocytes are yet another membrane abnormality that occurs within red blood cells. In this condition, several cells, instead of having a central area of pallor, possess a central “slit” of pallor. For this reason, they look like a mouth, or in particular, “kissing lips.” Even easier to remember is that they look like coffee beans. Take a look at them below:
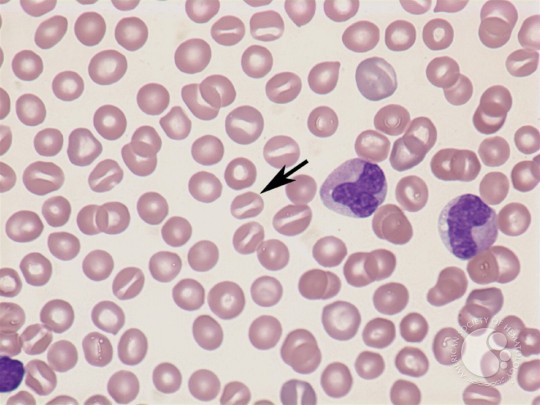
See? Coffee beans, kissing lips, you name it.
So why do these things arise? Basically, stomatocytes result from an increase in the volume of the red blood cell, and consequently a decrease in the surface area: volume ratio, due to some permeability defect in the membrane. (Contrast this with spherocytes and codocytes, which occur as a result of an increase in the surface area to volume ratio.)
The reason for the production of the mouth shaped slit is actually unknown at this point, but you can at least remember that stomatocytes are mouth shaped by picturing a mouth drinking water, similar to how water and salts move into stomatocytes due to their membrane defect.
Diseases/Conditions Associated with Stomatocytes
- Hereditary Stomatocytosis
- Hereditary stomatocytosis describes a number of inherited autosomal dominant human conditions which affect the red blood cell, in which the membrane or outer coating of the cell ‘leaks’ sodium and potassium ions.
- Osmosis leads to the red blood cell having a constant tendency to swell and burst. This tendency is countered by manipulating the flow of sodium and potassium ions. A ‘pump’ forces sodium out of the cell and potassium in, and this action is balanced by a process called ‘the passive leak’.
- In the hereditary stomatocytoses, the passive leak is increased and the cell becomes swamped with salt and water. The cell lyses and a haemolytic anaemia results.
- Alcoholism
- Liver Disease
- Rh Null Phenotype for Blood
- Individuals who possess the Rh null phenotype have osmotically fragile red cells, which take the form of stomatocytes.
- The osmotically fragile red cells are overfilled with water for their size even at water levels that would be normal for a normal erthyrocyte.
- Thus, they appear as stomatocytes in Rh null phenotypes.
- As an Artifact
- If only 10% of less of the cells seen are stomatocytes, then they are most likely artifacts.
Degmacytes (Bite Cells)
A degmacyte (aka “bite cell”) is an abnormally shaped red blood cell with one or more semicircular portions removed from the cell margin. These “bites” result from the removal of denatured hemoglobin by macrophages in the spleen. Glucose-6-phosphate dehydrogenase deficiency (G6PD), in which uncontrolled oxidative stress causes hemoglobin to denature and form Heinz bodies, is a common disorder that leads to the formation of bite cells.
The Heinz Bodies are seen as antigenic and are quickly phagocytosed. Because the Heinz Bodies are derivatives of hemoglobin, they are located inside the cell, and thus phagocytosis takes a significant “bite” out of the cell. This is the difference between the bite cell and the spherocyte, where only the membrane is destroyed.
Diseases Associated with Degmacytes:
- Glucose-6-phosphate Dehydrogenase Deficiency (G6PD)
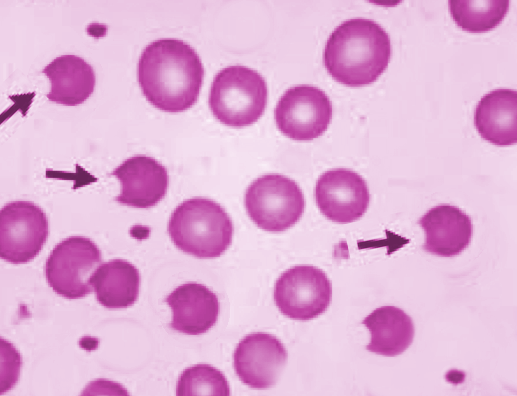
Drepanocytes (Sickle Cells)
This is one we all know. Sickle cell anemia is something we’re all familiar with. And it is again, a name that gives away what the cell is shaped like. You guessed it – the blade of a sickle, or a crescent. It is an autosomal recessive genetic disorder. The gene defect is a known mutation of a single nucleotide (GAG to GTG) of the β-globin gene, which results in glutamic acid being substituted by valine at position 6. This new hemoglobin is known as Hemoglobin S (HbS).
This is normally a benign mutation, causing no apparent effects on the secondary, tertiary, or quaternary structures of haemoglobin in conditions of normal oxygen concentration. What it does allow for, under conditions of low oxygen concentration, is the polymerization of the HbS itself. The deoxy form of haemoglobin exposes a hydrophobic patch on the protein between the E and F helices due to the valine. The hydrophobic side chain of the valine residue at position 6 of the beta chain in haemoglobin is able to associate with the hydrophobic patch, causing haemoglobin S molecules to aggregate and form fibrous precipitates.
In this case, as a result of the hydrophobic chains interacting, the red blood cell essentially folds in on itself, producing its unique shape.
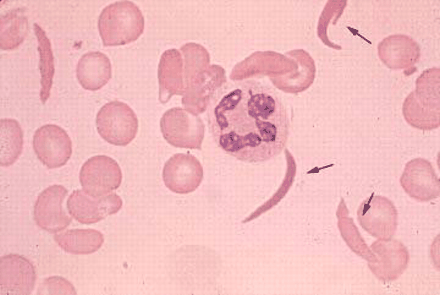
Poikilocytes of Trauma
Dacrocytes (Teardrop Cells)
Teardrop cells is such a cool name. And they are really shaped like teardrops. Thank you to whoever named these morphological abnormalities in cells. Dacrocytes can also be said to be pear shaped. They are usually characteristic of myelofibrosis, and seen with marrow disorders or marrow infiltrations, really because of improper production of blood cells from the bone marrow. In post-splenectomy patients, the number of dacrocytes drastically increases, since the spleen cannot remove the improperly formed cells.
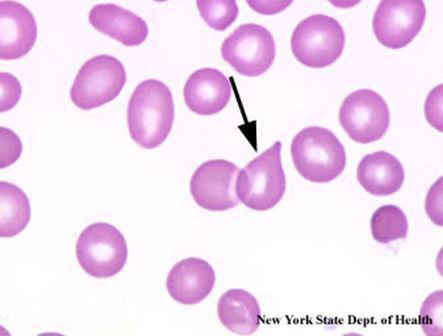
Diseases Associated with Dacrocytes:
- Myelofibrosis with myeloid metaplasia
- Myelofibrosis, also known as osteomyelofibrosis, is a rare bone marrow cancer.
- Beta Thalassemia Major
- Myelophthisic anemias
- Myelophthisic anemia (or myelophthisis) is a severe kind of anemia found in some people with diseases that affect the bone marrow. Myelophthisis refers to the displacement of hemopoietic bone-marrow tissue either by fibrosis, tumors or granulomas.
- Extramedullary Haematopoiesis
Schistocytes
A schistocyte is a fragmented portion of a red blood cell. It is literally a broken piece off a red blood cell. Obviously then, they are irregular shaped, jagged and have pointed extremities, that is, usually 2 pointy ends. There is no central pallor since they are simply fragments of red blood cells.
Schistocyte formation occurs as a result of mechanical destruction (fragmentation hemolysis) of a normal red blood cell. This occurs when there is damage to the blood vessel and a clot begins to form. The formation of the fibrin strands in the vessels occurs as part of the clot formation process. The red blood cells get trapped in the fibrin strands and the sheer force of the blood flow causes the red blood cell to break. The resulting fragmented cell is called the schistocyte.
They are thus very common in hemolytic anemias, obviously since RBCs are hemolysed and broken down in this condition.
Diseases Associated with Schistocytes:
- Microangiopathic hemolytic anemia (disseminated intravascular coagulation)
- Disseminated intravascular coagulation is an activation of the coagulation cascade which is usually a result of an increased exposure to tissue factor.
- The activation of the cascade leads to thrombi formation which causes an accumulation of excess fibrin formation in the intravascular circulation. The excess fibrin strands cause mechanical damage to the red blood cells resulting in schistocyte formation and also thrombocytopenia and consumption of clotting factors.
- Schistocyte values between .5% and 1% are usually suggestive of DIC.
- Thrombotic thrombocytopenic purpura
- Thrombotic thrombocytopenic purpura or TTP is caused by primary platelet activation. Thrombotic thrombocytopenic purpura leads to increased amounts of von Willebrand factor which then attach to activated platelets and mediate further platelet aggregation. Platelets end up being removed and the resulting fibrin strand formation remains. These fibrin strands along with the stress from the blood flow cause fragmentation of the red blood cells, leading to schistocyte formation.
- In TTP, a schistocyte count between 3-10% is common, but >1% is suggestive of the disease.
- Hemolytic uremic syndrome
- Haemolytic-uremic syndrome or HUS is haemolytic anaemia, acute kidney failure (uraemia), and thrombocytopenia.
- HUS is caused by E. coli bloody diarrhea and specific strains of shiga toxin. The bacteria in HUS cause damage to the endothelium which results in platelet activation and formation of microthrombi.
- Red cells get trapped in the fibrin strands of the microthrombi and become sheared by the force of blood flow leading to schistocyte formation.
- Severe burns
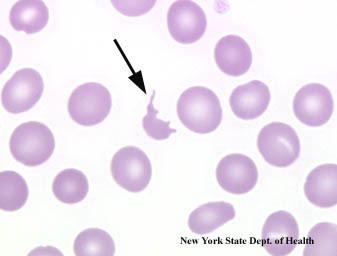
Keratocytes
Keratocyte formation may be associated with trauma, especially cellular damage from contact with fibrin strands within the microvasculature. Other processes that can contribute to microvascular injury include endotoxemia and antigen-antibody reactions. These forms of microangiopathy subsequently may lead to platelet aggregation, fibrin formation, and, ultimately, intravascular coagulation (DIC). As normal erythrocytes encounter a mesh of fibrin strands, they can become entrapped. Sometimes these cells are impaled on fibrin strands. Blood flow then pushes the cell against the strand of fibrin and the cell may bisect. Alternatively, the opposing sides of the cell may adhere to one another around the fibrin strand. When blood flow frees the cell, the opposing sides rejoin forming a pseudovacuole. Cells with pseudovacuoles are called “blister” cells or pre-keratocytes. When the vacuole ruptures (usually within minutes), the remaining cell resembles a helmet with straps or a horned cell that is designated a keratocyte. Prekeratocytes also can form via fusion of injured cell membranes (e.g., administration of doxorubicin6 and some iron deficiency anemias). These new cells are more fragile than the original parent cell and may rupture, forming a keratocyte.
A blister cell is seen below:
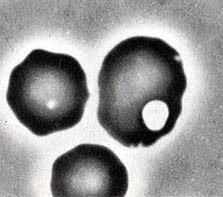
And here is a blister cell bursting:
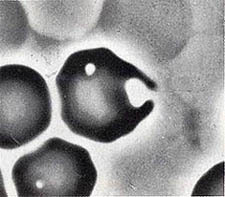
…Leading to the formation of the final Keratocyte:
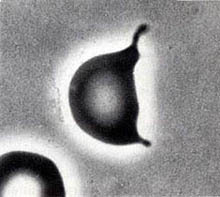
Semilunar Bodies
Semilunar bodies are types of poikilocytes caused by cell trauma. They are hypochromic, crescent shaped cells, also called ghost corpuscles/cells or phantom cells, with loss of all hemoglobin.This erythrocyte cell is an “empty” red cell membrane (the external structure or “covering”) that is left complete or intact after hemolysis, but the cell is dead. In some cases of AIHA/IMHA, semilunar bodies/ghost erythrocytes may be seen with intravascular hemolysis.
Semilunar bodies are types of poikilocytes caused by cell trauma. They are decolourised, crescent shaped cells, also called ghost corpuscles/cells or phantom cells. This erythrocyte cell is an “empty” red cell membrane (the external structure or “covering”) that is left complete or intact after hemolysis, but the cell is dead. In some cases of AIHA/IMHA, semilunar bodies/ghost erythrocytes may be seen with intravascular hemolysis.
Unique Cases of Poikilocytosis
These unique cases of poikilocytosis involve two important hemoglobinopathies: Hemoglobin SC disease and Hemoglobin C Crystals.
Hemoglobin SC Disease
Hemoglobin S-C disease is a hemoglobinopathy that causes symptoms similar to those of sickle cell disease, but milder. In this condition, only one allele of the autosomal sickle cell trait is present in patients. Thus, while the cells are not exactly “sickle cells” they still produce some abnormality in cell morphology.
This is how these cells look:
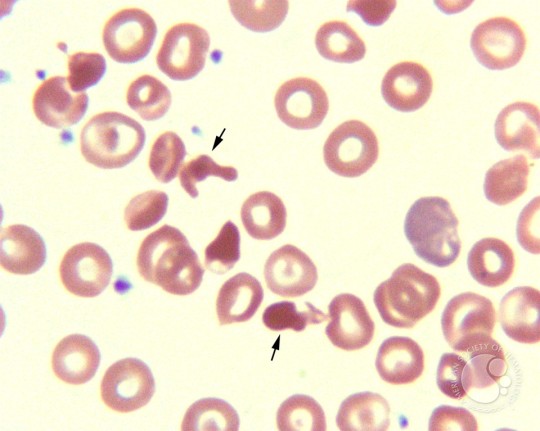
Thus, they can be described as “mitten-shaped” cells with 1-2 finger like projections.
Hemoglobin C Crystals
Hemoglobin C (abbreviated as Hb C or HbC) is an abnormal hemoglobin in which substitution of a glutamic acid residue with a lysine residue at the 6th position of the β-globin chain has occurred. This mutated form reduces the normal plasticity of host erythrocytes causing a hemoglobinopathy.
Target cells, microspherocytes and HbC crystals are found in a blood smear from a homozygous patient.
HbC crystals are the significant cell seen in this condition, and they look like this:
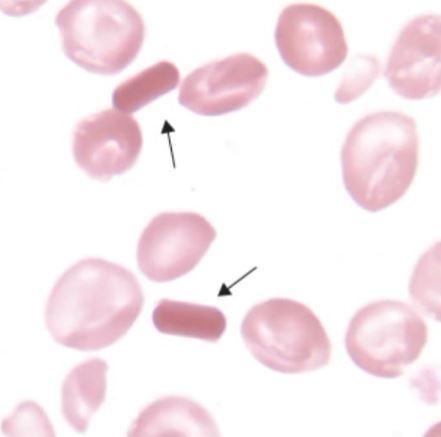
Yep. They look like pills. So whenever you see something like this in the blood film, along side target cells and microspherocytes, suspect HbC.
Elliptocytes/Ovalocytes
Red cells varying in shape from elongated to oval, and rich in hemoglobin, are called elliptocytes/ovalocytes. They can be seen in hereditary disorders, such as hereditary elliptocytosis, or in acquired disorders, such as iron defiency anemia, infectious anemias, thalassemia, and in newborn babies.
Elliptocytes: cigar-shaped
Ovalocytes: egg-shaped
That’s all guys! I’ve covered morphological abnormalities in this section. In the next topic, I will talk about inclusion bodies and improper arrangements of red blood cells. But for now, I hope this was useful!


NIce precise and well organized presentation.
LikeLike
Very helpful. I am glad how you explain the associates diseases/conditions with morphology.
LikeLiked by 1 person
Excellent. Than you.
LikeLike
Wonderful
LikeLike
Very, very helpful. Good job!
LikeLike
Thanks alot.it was so helpful.
LikeLike
That was great!! Thanks so much! I remembered some of the beauty of studying medicine ♡
LikeLike
very clear…….
LikeLike
Thanks. Now I can interpret my manual differential . Thank you . It was very helpful . I have MDS .
LikeLike
It is very nice…but how can I get it as a pdf ?
LikeLike
Nice all, its helpful to all, who study and practice related
LikeLike
wow excellent work thanx for your wonderful information
LikeLike
wow so great am lovin it
LikeLike
Has been very helpful for my haematology exam
Thank you!
LikeLike
Very helpful. Thanks
LikeLike
Your site is so Informative and Cool for Searching this medical thing.
Thanks For Sharing This Comment .
LikeLike
Nice
LikeLike
excelente y util
LikeLike
thank u. it was really helpful
LikeLike
Wow you have made heamatology nice for me
LikeLike
I like it. It is a very good wark.
LikeLike